Reports
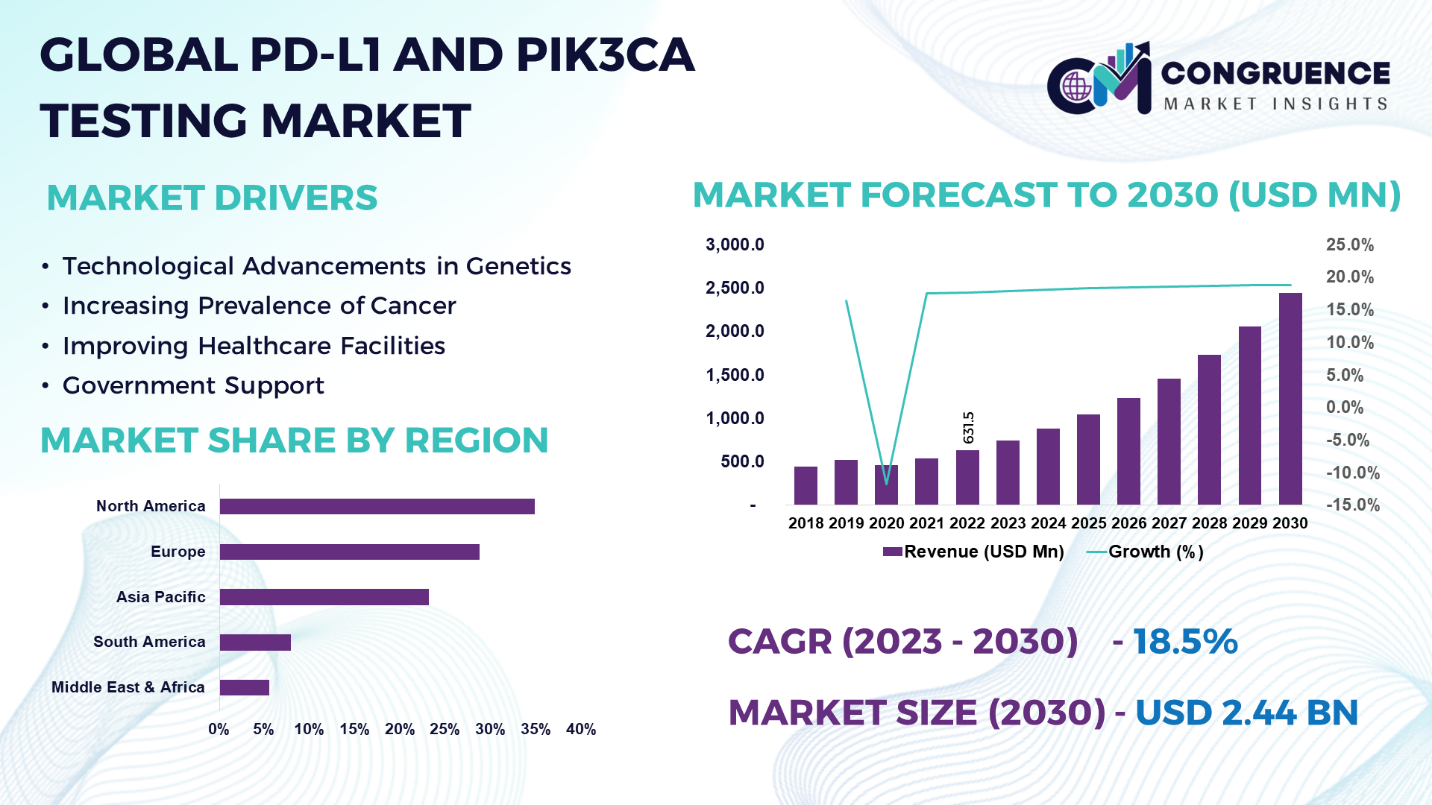
The Global PD-L1 and PIK3CA Testing Market was valued at USD 631.5 Million in 2022 and is anticipated to reach a value of USD 2,438.4 Million by 2030 expanding at a CAGR of 18.5% between 2023 and 2030.
PD-L1 Biomarker testing quantifies PDL1 levels on cancerous cells. A protein called PDL1 aids in preventing immune cells from attacking healthy cells in the body. Normally, your immune system targets foreign invaders like bacteria and viruses rather than your own healthy cells. There are some cancer cells with elevated PDL1 levels. By doing thus, the cancer cells are able to deceive the body's immune response and evade being recognized as dangerous, alien molecules. The procedures used for PD-L1 testing now use immunohistochemistry (IHC). Antibodies are utilized in immunohistochemistry (IHC) to identify proteins (antigens) in cells within a tissue segment (liver, pancreas, or heart, for example). The lipid kinase encoded by the PIK3CA gene plays a role in several signaling networks. These routes affect the development, division, and proliferation of cells. The PI3K-PTEN-AKT pathway, which is downstream of the EGFR and RAS-RAF-MAPK pathways, is activated by PIK3CA mutations. Independent of RAS or RAF mutations, PIK3CA mutations have the potential to induce oncogenic transformation. PIK3CA mutations are more common in breast, endometrial, and colon cancers, whereas, in lung cancer and other cancer types, they are less prevalent. When PIK3CA mutations are present, patients with breast, endometrial, or colon cancer have a worse prognosis and don't respond to certain treatments.
PD-L1 and PIK3CA Testing Market Major Driving Forces
Technological Advancements in Genetics: Technological developments in genomic profiling will probably improve the precision and efficacy of PD-L1 and PIK3CA testing. Comprehensive tumor genome profiling will be made possible by the combination of next-generation sequencing with other molecular diagnostic tools, giving clinicians the ability to choose the best course of action for each patient.
Increasing Prevalence of Cancer: Cancer incidences are increasing worldwide as a result of a hectic schedule and an unhealthy lifestyle. Improper sleep cycle, fast and junk food, polluted water and air are all contributing to an increase in cancer incidence, which pushes the demand for PD-L1 and PIK2CA testing.
Improving Healthcare Facilities: The growing number of hospitals and improved healthcare facilities around the world have resulted in better cancer therapy, popularizing cancer treatment, and thereby driving the market for these tests.
Government Support: Governments everywhere are placing a great deal of attention on increasing the number of cancer patients receiving treatment. The high death rate from diseases such as cancer has drawn attention from all around the world to this problem's solution. As a result, the PD-L1 and PIK3CA testing markets have significant state support.
PD-L1 and PIK3CA Testing Market Key Opportunities
Increasing Utilization in Various Cancers: Testing for PD-L1 and PIK3CA is not restricted to any particular kind of cancer. There is room to grow the applicability of these tests to a wider range of tumors so that more patients can take advantage of individualized and focused treatment plans.
Immunotherapy Advances: Immunotherapy improvements, particularly immune checkpoint inhibitors, underscore the importance of precise PD-L1 testing. Identifying patients with high PD-L1 expression assists oncologists in determining the efficacy of immunotherapy, allowing for a more tailored and successful approach.
Customization and Personalization: Customers are increasingly looking for specialized testing solutions. Manufacturers of PD-L1 and PIK3CA testing can provide these tests depending on each patient's unique molecular profile. This trend improves the precision and accuracy of test results, opening up potential in premium and specialist industries.
PD-L1 and PIK3CA testing market Key Trends
· There is an increasing need for PD-L1 and PIK3CA testing due to the emphasis on customized treatment plans.
· Immunotherapies, especially immune checkpoint inhibitors, are becoming more widely used.
· The clinical uses for PD-L1 and PIK3CA testing are growing as a result of ongoing research and development.
· Testing procedures are constantly being improved, with liquid biopsies and next-generation sequencing among them.
· The creation of complementary diagnostics that work in tandem with focused treatments.
· The global spread of PD-L1 and PIK3CA testing services is partly attributed to the growing healthcare infrastructure in emerging economies.
Region-wise Market Insights
North America accounted for the largest market share at 34.8% in 2022 whereas, Asia Pacific is expected to register the fastest growth, expanding at a CAGR of 19.4% between 2023 and 2030.
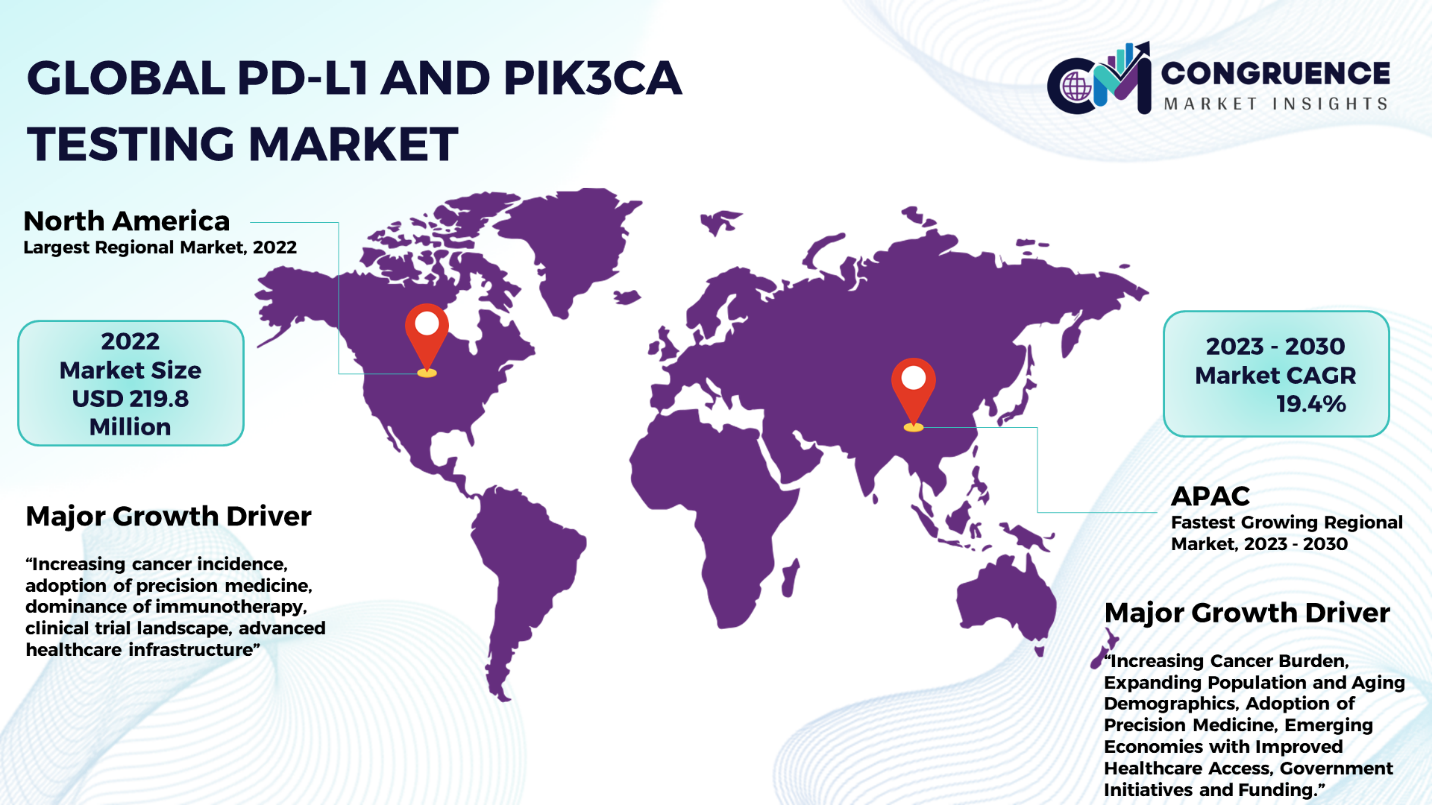
North America has the greatest market share of 34.8% in the PD-L1 and PIK3CA testing market, accounting for USD 219.8 million. The growing incidence of cancer in North America has prompted testing for PD-L1 and PIK3CA. The need for tailored treatment strategies, the rise of precision medicine, and our growing understanding of the molecular origins of cancer are boosting demand in this field. Furthermore, the clinical trial landscape in North America is quite robust, with many trials investigating novel medicines and combinations. PD-L1 and PIK3CA tests are in great demand due to their crucial role in patient classification for clinical trials. Asia-Pacific region, on the other hand, is projected to register the highest CAGR of 19.4% of the course of forecasted period. A growing number of people in the Asia-Pacific area are getting cancer as a result of environmental factors, changing lifestyles, and aging populations. The Asia-Pacific region's huge and aging population is a factor in the rising cancer prevalence. There is a greater chance of cancer occurring as people age. The trend of precision medicine practice adoption in Asia-Pacific is developing due to advancements in healthcare infrastructure and rising awareness. The economies of many Asia-Pacific nations are expanding, and their infrastructure for providing healthcare is getting better. Better access to modern diagnostics, such as PD-L1 and PIK3CA testing, is the outcome of this.
Market Competition Landscape
Precision medicine-focused startups, pharmaceutical corporations, and well-established diagnostic companies make up the competitive landscape of the PD-L1 and PIK3CA testing market, which is constantly changing. Due to their proficiency in companion diagnostics development and molecular diagnostics, major firms including Agilent Technologies, Thermo Fisher Scientific, and Roche Diagnostics have substantial market shares. These businesses frequently work strategically along with industry titans in the pharmaceutical industry to jointly create companion diagnostics and targeted medicines. Leading pharmaceutical firms like AstraZeneca, Merck & Co., and Bristol Myers Squibb also play a significant role in promoting innovation in the PD-L1 and PIK3CA testing fields as they look to identify patient populations that might benefit from the targeted treatments and immunotherapies that they offer. Among the leading companies in the market are:
· Roche Diagnostics
· Thermo Fisher Scientific
· Agilent Technologies
· Bristol Myers Squibb
· Merck & Co.
· AstraZeneca
· Qiagen
· Illumina
· Foundation Medicine (Roche)
· Guardant Health
· Genentech (Roche Group)
· Abbott Laboratories
· Guardant360 (Guardant Health)
· Bio-Rad Laboratories
· Sysmex Corporation
Roche Diagnostics is one of the top firms in the world that develops and markets PD-L1 testing solutions. Companion diagnostics are tools that help identify patients who are a good fit for immunotherapy, owing to the company's dedication to advancing precision medicine. A major force in the diagnostics and life sciences industries is Thermo Fisher Scientific. In addition to molecular diagnostic assays, the company offers a variety of technologies and instruments. Precision medicine skills are expanded by its engagement in the development of PD-L1 and PIK3CA testing tools.
|
Report Attribute/Metric |
Details |
|
Market Revenue in 2022 |
USD 631.5 Million |
|
Market Revenue in 2030 |
USD 2,438.4 Million |
|
CAGR (2023 – 2030) |
18.5% |
|
Base Year |
2022 |
|
Forecast Period |
2023 – 2030 |
|
Historical Data |
2018 to 2022 |
|
Forecast Unit |
Value (US$ Mn) |
|
Key Report Deliverable |
Revenue Forecast, Growth Trends, Market Dynamics, Segmental Overview, Regional and Country-wise Analysis, Competition Landscape |
|
Segments Covered |
· By Type of Test (PD-L1 Testing, PIK3CA Testing) · By Cancer Type (Lung Cancer, Breast Cancer, Colorectal Cancer and Others) · By End-User (Hospitals and Clinics, Diagnostic Laboratories, Research Institutions) |
|
Geographies Covered |
North America: U.S., Canada and Mexico Europe: Germany, France, U.K., Italy, Spain, and Rest of Europe Asia Pacific: China, India, Japan, South Korea, Southeast Asia, and Rest of Asia Pacific South America: Brazil, Argentina, and Rest of Latin America Middle East & Africa: GCC Countries, South Africa, and Rest of Middle East & Africa |
|
Key Players Analyzed |
Roche Diagnostics, Thermo Fisher Scientific, Agilent Technologies, Bristol Myers Squibb, Merck & Co., AstraZeneca, Qiagen, Illumina, Foundation Medicine (Roche), Guardant Health, Genentech (Roche Group), Abbott Laboratories, Guardant360 (Guardant Health), Bio-Rad Laboratories, Sysmex Corporation |
|
Customization & Pricing |
Available on Request (10% Customization is Free) |
