Reports
The Global Chronic Fatigue Syndrome Therapeutics Drug Market was valued at USD 297.1 Million in 2023 and is anticipated to reach a value of USD 416.8 Million by 2031 expanding at a CAGR of 4.4% between 2024 and 2031.
The global chronic fatigue syndrome (CFS) therapeutic drug market plays a crucial role in the healthcare industry. It finds solutions to manage the complicated symptoms of this refractory problem. Chronic fatigue syndrome, also called myalgic encephalomyelitis (ME/CFS), is a debilitating condition which people suffer due to presence of persistent fatigue. It reduces person's ability to do their routine activities. Patients also tells a variety of symptoms that are beyond fatigue, such as cognitive dysfunction, muscle pain and sleeping disorder. It leads to the multidimensional difficulties for healthcare professionals. Increasing role of research in the search for underpinning cause of CFS drove the drug development efforts for attention of effective therapeutics and symptom relief for better well-being. The research field of CFS includes the discovery and development of newly formulated drugs and treatment methods by pharmaceutical companies, biotech firms, and research institutions which work on different CFS pathologies components. As the society becomes more CFS informed, and diagnostic criteria becomes more specific, the global pharmaceutical market fully expects a substantial growth in the CFS therapeutic drugs stocking implying hope for improved management of sufferers worldwide.
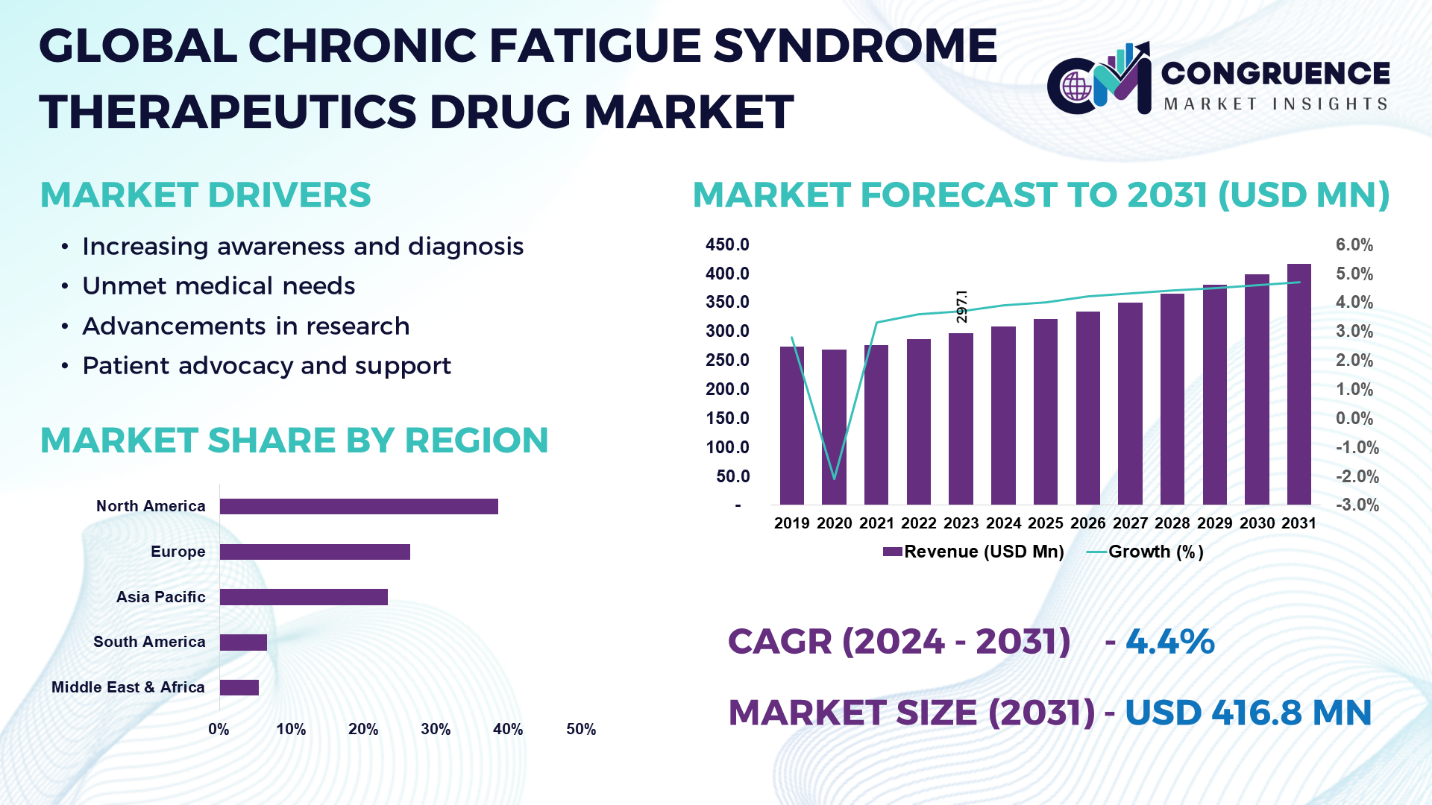
Chronic Fatigue Syndrome Therapeutics Drug Market Major Driving Forces
Increasing Awareness and Diagnosis: From the rise of CFS awareness among healthcare professionals and the general public has been seen the development of superior diagnosis rates. Although CFS is not widely known among the public, more and more people are being diagnosed with it, which subsequently corresponds to grow the demand for effective drug management to abate symptoms and improve patients’ quality of life.
Unmet Medical Needs: Disregarding the fact that chronic fatigue syndrome is a disability, the lack of drugs that are approved as being specifically looked for this disorder is astonishing. This thus creates a withdrawal of considerable medical need which lures pharmaceutical giants as well as researchers to divert their efforts to the investigation of novel therapeutics, which get at the root cause of the disease.
Advancements in Research: With the consistently-growing research into the pathology of CFS, it becomes deeper understanding of the pathological aspects of the condition. This knowledge has made it possible for scientists to spot drug targets and the best approaches to treat CFS, which has contributed immensely to the development of new CFS therapeutic strategies.
Patient Advocacy and Support: CFS patient advocacy groups and other organizations act as key agents in increasing the population understanding of the illness, and in they call for researches’ funding and treatment improvement. Such actions include recruitments of more professionals in the field of CFS and thus create more research space as well as the implementation of various projects leading to the development of CFS-relevant drugs.
Chronic Fatigue Syndrome Therapeutics drug Market Key Opportunities
Targeted Therapies: For CFS researchers, this is the dawn of an era, offering the prospect to create patient-specific or cluster-specific treatments. Similar to CFS, biomarkers and molecular pathways can also be identified and drugs are proposed to be different as compared to the existing ones (conventional treatment) as drug got designed that involves treating the underlying mechanism in a more effective way, thus paving the wave to the improved treatment outcomes.
Combination Therapies: Combination therapies, which attack multiple pathways involved in CFS pathogenesis, is a promising approach in eliciting an improved effect on the treatment of CFS and in consideration of the impression of varied symptoms of CFS as represented by heterogeneous signs. The drug combinations with related mechanisms of actions are producing a synergy effect that leads to the maximizing of effects and minimizing of any adverse reactions.
Repurposing Existing Drugs: The development of drugs through a process of repurposing existing drugs for other indications brings with it the potential for cost-effective drug development for CFS. Moreover, this often reduces the time needed for drug approval. The big Pharma may depend on these drugs’ safety and effectiveness data that are already available, fast tracking the route to market, and reducing the costs for development.
Chronic Fatigue Syndrome Therapeutics Drug Market Key Trends
· Redirecting research funds to CFS pathogenesis causes elucidation and finding drug targets.
· Continuous growth of the investigation into the exploitation of drugs, which were already used in the treatment, for CFS therapy.
· Advancement in treatment based on personalized strategies for effective customizing treatments according to an individual patient pattern.
· CFS third mechanism leads development of the combination therapies attacking different pathways in CFS.
· Implementing the digital health technologies, such as telehealth monitoring and symptom management, that incorporate CFS remotely.
· Stronger regulation policies and financial investment in drugs for rare conditions (e.g. Chronic Fatigue Syndrome).
· Patient-centric solutions for improved drug delivery.
· Partnerships development with the pharmaceutical company and advocacy organizations so as to boost the research and awareness campaigns on this subject.
· Stratification of patient-reported outcomes and real-world evidence into future clinical trial designs across CFS drugs.
· Investigating of the possibilities of CFS complementary and alternative therapies as an adjunct treatment for CFS.
Region-wise Market Insights
North America accounted for the largest market share at 38.5% in 2023 whereas, Asia Pacific is expected to register the fastest growth, expanding at a CAGR of 5.1% between 2024 and 2031.
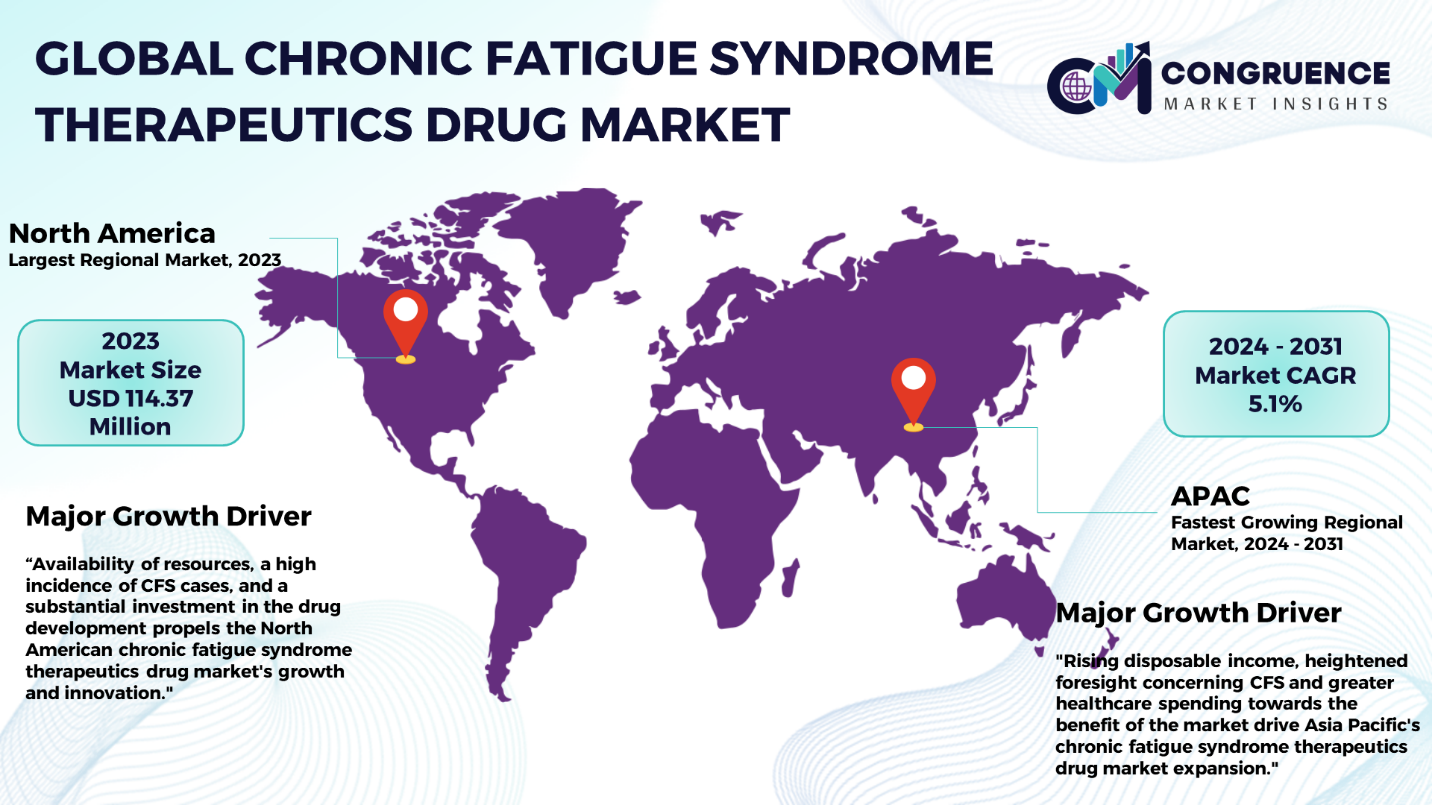
The study of the worldwide regional situation of the drugs of chronic fatigue syndrome therapeutics (CFS) reports many different patterns of change and dynamics in different countries regions. The drug development and clinical trials in North America, especially in the United States, are among the leaders as a result of the availability of resources, a high incidence of CFS cases, and a substantial investment in the drug development. The area enjoys a tight-knit regulatory system with strong input from patient advocacy organizations that helps to make pharma companies innovative and to establish fruitful collaborations between research institutions, healthcare providers, and the pharmaceutical industry itself. Europe, including the countries such as the United Kingdom, Germany, and Scandinavia, are seeking treatment options for CFS too. They have projects that are innovating medicine and allowing a person to get treatment. In Asia Pacific there is heightened foresight concerning CFS and greater healthcare spending towards the benefit of the market in countries such as Japan, Australia, and South Korea for instance. Adding on to this, regulatory hurdles and inadequate resources that are at the disposal of the country hinder market knowledge dissemination of the product in some other regions as well. Thus, the regional map clearly demonstrates the importance of applying focused strategies as well as collaborative efforts to cover the dynamic individual requirements in any local CFS therapeutics drugs market.
Market Competition Landscape
Within the fiercely competitive CFS therapy drugs market where pharma companies are battling to capture market shares through the harnessing of creative healthcare technologies, breakthrough drug development is the key word. Major actors spend a lot of resources in clinical trials to prove efficiency and safety of their drug candidates thus the strategic alliances and acquisitions are the key tools they utilize to advance. Furthermore, research by smaller biotechnology companies and academician not only increase competition but also leads to the invention of new drug discovery efforts. Competition becomes much more deep seated along with the higher need for the efficient CFS cure, which in turns keeps the sector advancing and in pursuit of the collaborations. Prominent players in the market include:
· Pfizer Inc.
· Novartis AG
· Johnson & Johnson
· Merck & Co., Inc.
· GlaxoSmithKline plc
· AstraZeneca plc
· Eli Lilly and Company
· AbbVie Inc.
· Bristol Myers Squibb
· Amgen Inc.
· Biogen Inc.
· Gilead Sciences, Inc.
· Vertex Pharmaceuticals Incorporated
· Regeneron Pharmaceuticals, Inc.
· Moderna, Inc.
|
Report Attribute/Metric |
Details |
|
Market Revenue in 2023 |
USD 297.1 Million |
|
Market Revenue in 2031 |
USD 416.8 Million |
|
CAGR (2024 – 2031) |
4.4% |
|
Base Year |
2023 |
|
Forecast Period |
2024 – 2031 |
|
Historical Data |
2019 to 2023 |
|
Forecast Unit |
Value (US$ Mn) |
|
Key Report Deliverable |
Revenue Forecast, Growth Trends, Market Dynamics, Segmental Overview, Regional and Country-wise Analysis, Competition Landscape |
|
Segments Covered |
· By Disease Severity (Mild, Moderate, Severe) · By Route of Administration (Oral, Injectable, Topical) · By Patient Age Group (Adults, Pediatrics) · By End User (Hospitals, Clinics, Ambulatory Surgical Centers) · By Treatment Approach (Symptomatic Relief, Disease-modifying Therapy) · By Drug Development Stage (Preclinical, Clinical) · By Mechanism of Action (Antiviral, Immunomodulatory, Analgesic) · By Product Type (Branded Drugs, Generic Drugs) |
|
Geographies Covered |
North America: U.S., Canada and Mexico Europe: Germany, France, U.K., Italy, Spain, and Rest of Europe Asia Pacific: China, India, Japan, South Korea, Southeast Asia, and Rest of Asia Pacific South America: Brazil, Argentina, and Rest of Latin America Middle East & Africa: GCC Countries, South Africa, and Rest of Middle East & Africa |
|
Key Players Analyzed |
Pfizer Inc., Novartis AG, Johnson & Johnson, Merck & Co., Inc., GlaxoSmithKline plc, AstraZeneca plc, Eli Lilly and Company, AbbVie Inc., Bristol Myers Squibb, Amgen Inc., Biogen Inc., Gilead Sciences, Inc., Vertex Pharmaceuticals Incorporated, Regeneron Pharmaceuticals, Inc., Moderna, Inc. |
|
Customization & Pricing |
Available on Request (10% Customization is Free) |
