Reports
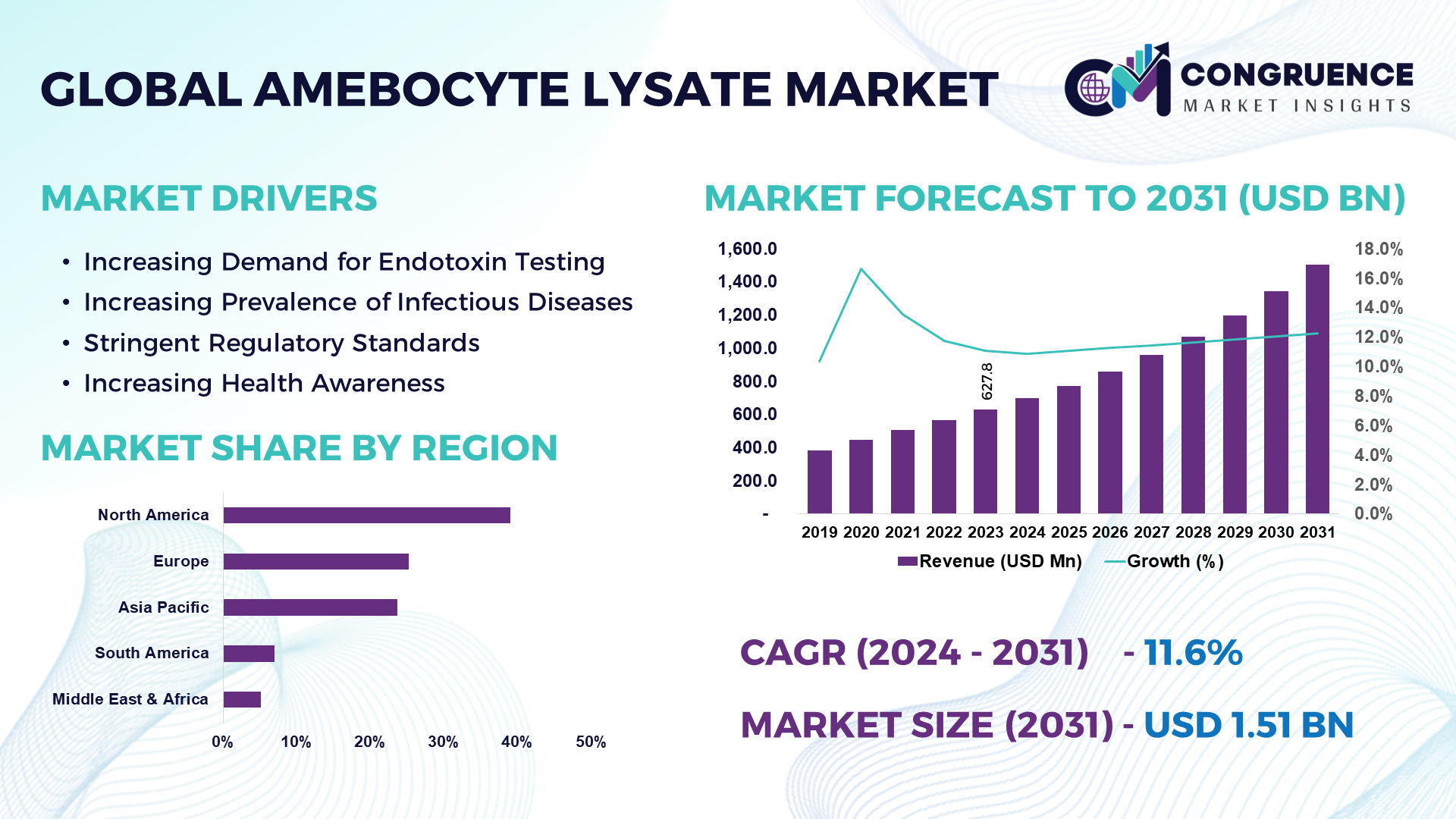
The Global Amebocyte Lysate Market was valued at USD 627.8 Million in 2023 and is anticipated to reach a value of USD 1,505.0 Million by 2031 expanding at a CAGR of 11.6% between 2024 and 2031.
Amebocyte lysate is an extract derived from the blood cells (amebocytes) of the horseshoe crab. They are similar to white blood cells in humans and play a vital role in the crab’s immune system. Amebocyte lysate is used to detect bacterial endotoxins. Bacterial endotoxin testing, commonly knowns as amebocyte lysate testing, is used to determine the presence and concentration of bacterial endotoxin in the environment. Amebocyte lysate contains enzymes that react with endotoxins, this reaction is the basis for the limulus amebocyte lysate (LAL) test, used to detect endotoxins in pharmaceuticals, medical devices, water and food products. The market is classified into limulus ameobcyte lysate, and tachlypleus amebocyte lysate. The market growth is influenced by factors such as increasing demand for endotoxin testing, increasing prevalence of infectious diseases, stringent regulatory standards, and increasing health awareness. Amebocyte lysate plays a crucial role in ensuring the safety of various products by detecting harmful bacterial endotoxins.
To learn more about this report, request a free sample copy
Amebocyte Lysate Market Major Driving Forces
Increasing Demand for Endotoxin Testing: The growing concern over the safety of pharmaceuticals and medical devices is influencing the need for endotoxin testing. Amebocyte lysate is a vital reagent for endotoxin detection and quantification.
Increasing Prevalence of Infectious Diseases: With the increasing global population, the spread of infectious diseases is becoming a major concern. As sepsis instances and infectious illness prevalence rise, there is a greater need for thorough endotoxin testing in healthcare settings, which is driving the market growth.
Stringent Regulatory Standards: Regulatory bodies such as FDA and EMA implement stringent endotoxin testing for pharmaceuticals and medical devices is driving the need for amebocyte lysate products. The pharmaceutical and medical devices companies operate under stringent regulatory frameworks that mandate rigorous testing for endotoxins.
Increasing Health Awareness: The growing awareness of the importance of drug and device safety among consumers and healthcare professionals boosts the demand for reliable endotoxin testing. This awareness is leading to a greater focus on the use of f LAL testing to detect and quantify endotoxins in these products.
Amebocyte Lysate Market Key Opportunities
Expansion of Healthcare Infrastructure: The increasing investments in expansion of healthcare infrastructure is expected to create significant opportunities for market growth. With the improvement in healthcare infrastructure globally, the demand for medical devices and pharmaceuticals rises, subsequently boosting the market for amebocyte lysate.
Advancements in Testing Methods: Ongoing advancements in testing methods, such as introduction of automated systems and rapid detection kits improves testing efficiency and accuracy. Furthermore, development of more efficient and accurate amebocyte lysate testing technology, boosting market growth.
Expansion in Emerging Markets: Expanding into emerging economies is expected to provide lucrative opportunities for market players. Improving healthcare infrastructure, increasing healthcare spending, growing awareness in emerging economies are anticipated to drive market expansion in these regions.
Amebocyte Lysate Market Key Trends
· The shift towards recombinant alternatives is becoming more popular as a substitute for conventional LAL assays
· The increasing demand for endotoxin testing due to growing concern over the safety of pharmaceuticals and medical devices
· The spread of infectious diseases is becoming a major concern with the growing global population is creating greater need for thorough endotoxin testing in healthcare settings
· Ongoing developments in biotechnology and healthcare fields fueling demand for LAL testing to ensure product safety and efficacy
· Increased healthcare spending is fueling investments in advanced diagnostic tools and technologies, including LAL testing
· Growth in pharmaceutical production and stricter quality control regulations are driving the need for LAL products in pharmaceutical manufacturing processes
· The development of portable and quick LAL test kits for point-of-care endotoxin detection to meet the needs of healthcare settings are gaining traction
Region-wise Market Insights
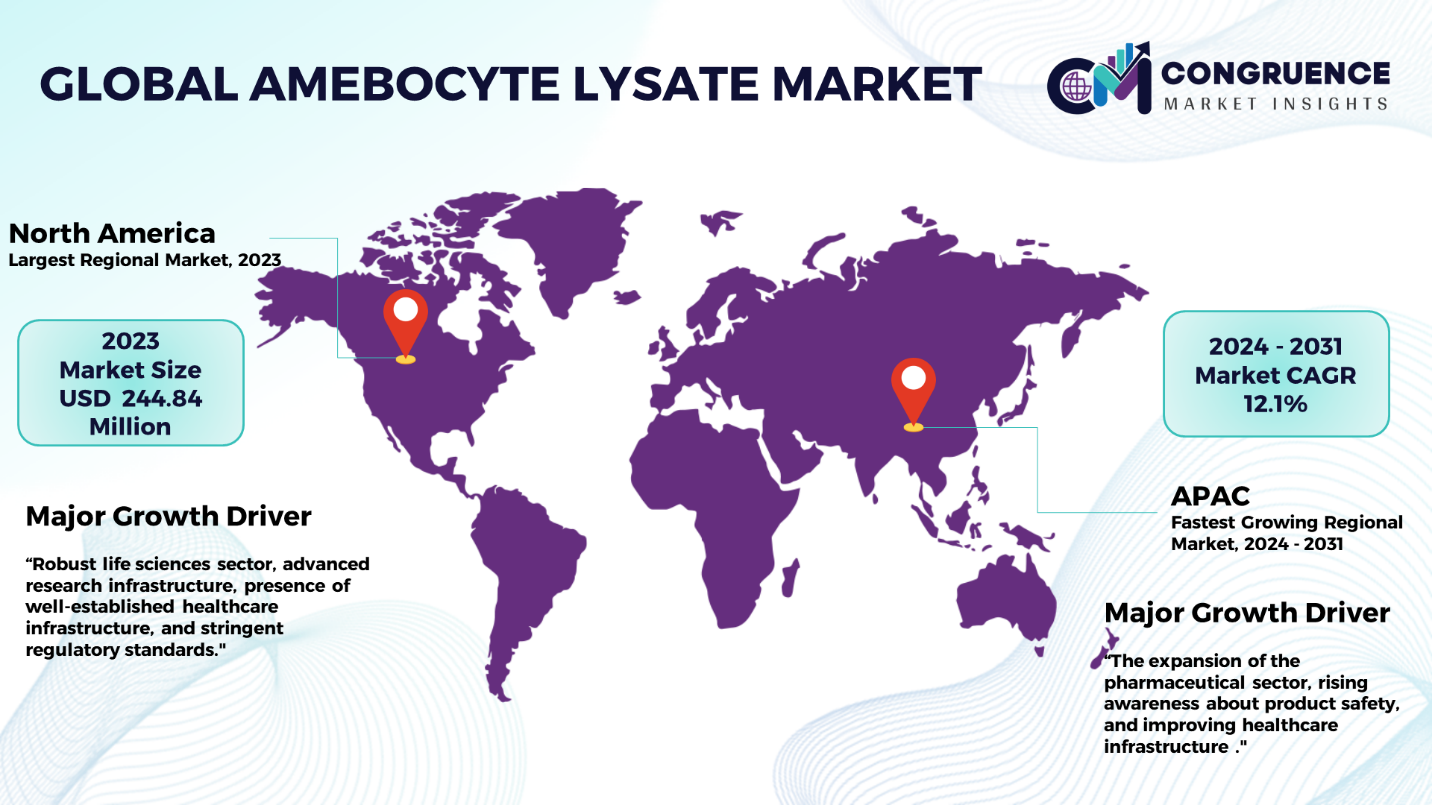
North America accounted for the largest market share at 39.0% in 2023 whereas, Asia Pacific is expected to register the fastest growth, expanding at a CAGR of 12.1% between 2024 and 2031.
To learn more about this report, request a free sample copy
North America dominates the global amebocyte lysate market due to robust life sciences sector, advanced research infrastructure, presence of well-established healthcare infrastructure, and stringent regulatory standards in the region. Furthermore, the region’s well-established pharmaceutical sector, and growing investments in the development of advanced biologics and medical devices are some significant factors drives market growth. In Europe, the market is driven by robust healthcare infrastructure, emphasis on research and development, and stringent regulatory standards. In Asia-Pacific, the market is characterized by the expansion of the pharmaceutical sector, rising awareness about product safety, and improving healthcare infrastructure which has created an increasing demand for amebocyte lysates. The Middle East and Africa has been witnessing a growing demand for amebocyte lysate due to increasing healthcare investments whereas, in South America, the market is influenced by economic factors, with improving healthcare infrastructure and increasing focus on pharmaceutical manufacturing.
Market Competition Landscape
The global amebocyte lysate market is characterized by high degree of competition among a large number of manufacturers. Key players in the amebocyte lysate market engage in strategies aimed at gaining a competitive edge. These strategies include product innovation, design differentiation, and the incorporation of sustainable and eco-friendly materials to meet evolving consumer preferences. Established brands leverage their reputation for quality and reliability to maintain market share, while newer entrants focus on disruptive innovations and unique selling propositions.
Key players in the global amebocyte lysate market implement various organic and inorganic strategies to strengthen and improve their market positioning. Prominent players in the market include:
· Zhanjiang Bokang Marine Biological Co., Ltd.
· Lonza Group AG
· Pacific BioLabs
· Associates of Cape Cod, Inc.
· Seikagaku Corporation
· Bio-Synthesis Inc.
· Biogenuix
· Thermo Fisher Scientific, Inc.
· Sartorius AG
· Genscrip
· Charles River Laboratories
· Merck KGaA
· AstraZeneca
· Xiamen Bioendo Technology Co., Ltd.
|
Report Attribute/Metric |
Details |
|
Market Revenue in 2023 |
USD 627.8 Million |
|
Market Revenue in 2031 |
USD 1,505.0 Million |
|
CAGR (2024 – 2031) |
11.6% |
|
Base Year |
2023 |
|
Forecast Period |
2024 – 2031 |
|
Historical Data |
2019 to 2023 |
|
Forecast Unit |
Value (US$ Mn) |
|
Key Report Deliverable |
Revenue Forecast, Growth Trends, Market Dynamics, Segmental Overview, Regional and Country-wise Analysis, Competition Landscape |
|
Segments Covered |
· By Type (Limulus Ameobcyte Lysate, and Tachlypleus Amebocyte Lysate) · By Application (Drug Testing, Clinical Diagnostics, Quality Control, Environmental Testing, and Others) · By End-user (Pharmaceutical Companies, Biotechnology Companies, Contract Research Organizations (CROs), Research and Academic Institutions) |
|
Geographies Covered |
North America: U.S., Canada and Mexico Europe: Germany, France, U.K., Italy, Spain, and Rest of Europe Asia Pacific: China, India, Japan, South Korea, Southeast Asia, and Rest of Asia Pacific South America: Brazil, Argentina, and Rest of Latin America Middle East & Africa: GCC Countries, South Africa, and Rest of Middle East & Africa |
|
Key Players Analyzed |
Zhanjiang Bokang Marine Biological Co., Ltd.,Lonza Group AG,Pacific BioLabs,Associates of Cape Cod, Inc.,Seikagaku Corporation,Bio-Synthesis Inc.,Biogenuix,Thermo Fisher Scientific, Inc.,Sartorius AG,Genscrip,Charles River Laboratories,Merck KGaA,AstraZeneca, and Xiamen Bioendo Technology Co., Ltd. |
|
Customization & Pricing |
Available on Request (10% Customization is Free) |
