Reports
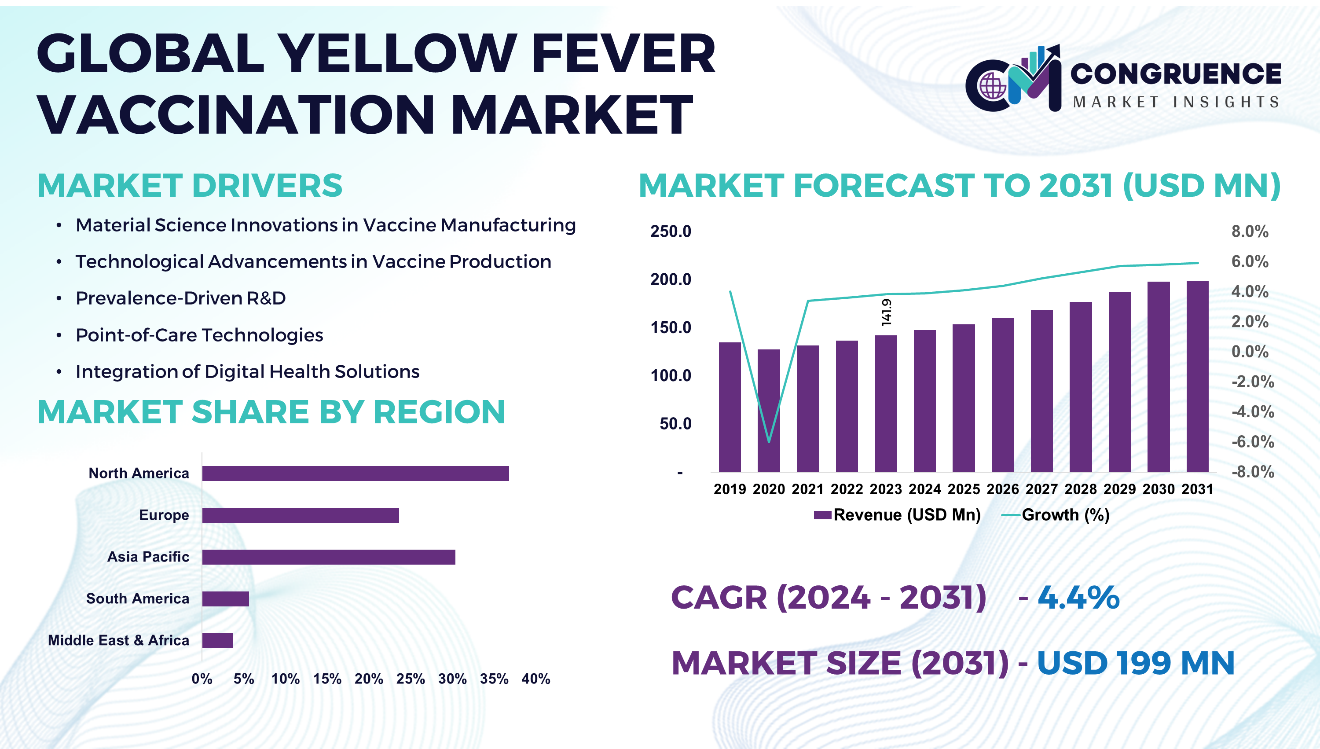
The Global Yellow Fever Vaccination Market was valued at USD 141.9 Million in 2023 and is anticipated to reach a value of USD 199.0 Million by 2031 expanding at a CAGR of 4.4% between 2024 and 2031.
Yellow fever vaccines are pivotal in addressing the escalating prevalence of this viral illness, particularly in regions where it is endemic. With the surge in global travel, there is a heightened risk of yellow fever spreading to previously unaffected areas. Medical technology innovations have significantly contributed to the treatment and prevention of yellow fever by facilitating swift diagnosis, vaccine development, and proficient patient care. Although AI holds potential in various medical domains, its direct application in yellow fever treatment remains somewhat limited. Nevertheless, AI-driven data analytics and modelling can offer valuable insights for predicting outbreaks, optimizing vaccination, focusing on molecular intricacies. Concurrently, advancements in material science have bolstered the manufacturing processes of yellow fever vaccines, ensuring efficacy, and stability. By harnessing these multidisciplinary approaches, healthcare practitioners and researchers can continue making substantive progress in combatting yellow fever and upholding health care standards.
Yellow Fever Vaccination Market Major Driving Forces
Material Science Innovations in Vaccine Manufacturing: Advances in material science have led to the development of novel vaccine formulations and delivery systems, enhancing stability. These innovations contribute to increased vaccine availability, particularly in resource-limited settings where cold-chain infrastructure stays inadequate.
Technological Advancements in Vaccine Production: Technological advancements such as recombinant DNA technology and cell culture techniques have revolutionized vaccine production, enabling more efficient and scalable manufacturing process for yellow fever vaccines. This has resulted in increased production capacity, thereby addressing growing demand and supply challenges.
Prevalence-Driven R&D: The growing prevalence of yellow fever, coupled with the threat of urbanization and climate change influencing disease transmission dynamics, drives R&D efforts aimed at improving existing vaccines and developing next-gen vaccine candidates.
Point-of-Care Technologies: The increasing prevalence of yellow fever underscores the need for rapid and accurate diagnostic tools to facilitate early detection and intervention. Point-of-care technologies leveraging material science innovations, such as paper-based microfluidic devices and nano-particle-based assays, enable decentralized testing and real-time surveillance in resource-limited settings, thereby supporting timely public health response and vaccinations.
Integration of Digital Health Solutions: Digital health solutions powered by advanced technologies, including AI, big data analytics, and mobile health platforms, are being utilized to monitor disease prevalence, track vaccine coverage, and optimize immunization strategies for yellow fever. These innovative approaches enhance surveillance capabilities, inform targeted vaccination campaigns, and improve overall disease control efforts in endemic regions.
Yellow Fever Vaccination Market Key Opportunities
Development of Next-Generation Vaccines: Continued R&D efforts offers opportunities to develop next-gen yellow fever vaccine with improve safety, efficacy, and scalability. This includes exploring novel vaccine platforms, and delivery systems to enhance immune response and overcome existing challenges associated with vaccine production and distribution.
Innovation in Vaccine Delivery: Opportunities exist to innovate in vaccine delivery mechanisms to overcome logistical challenges and improve vaccine accessibility in remote and resource-limited settings. This includes exploring novel delivery routes, such as intradermal or oral administration, and developing heat-stable formulations to reduce reliance on cold-chain infrastructure.
Integration with Other Health Initiatives: Integration of yellow fever vaccination with other health initiatives, such as routine immunization, and vector control interventions, offers growth opportunities to the market. This helps optimize disease control efforts and strengthens overall health systems' resilience.
Yellow Fever Vaccination Market Key Trends
· Incorporating digital surveillance, tracking, and vaccination management technologies enhances yellow fever control efforts.
· Advancements in gene editing offer potential for developing a more efficient and targeted yellow fever vaccine.
· Nanoparticle-based vaccine delivery systems improve the immunogenicity and stability of yellow fever vaccines, advancing efficacy.
· Telehealth platforms facilitate remote consultation, and monitoring, enhancing allow fever prevention and management strategies.
Region-wise Market Insights
North America accounted for the largest market share at 36.7% in 2023 whereas, Asia Pacific is expected to register the fastest growth, expanding at a CAGR of 4.6% between 2024 and 2031.
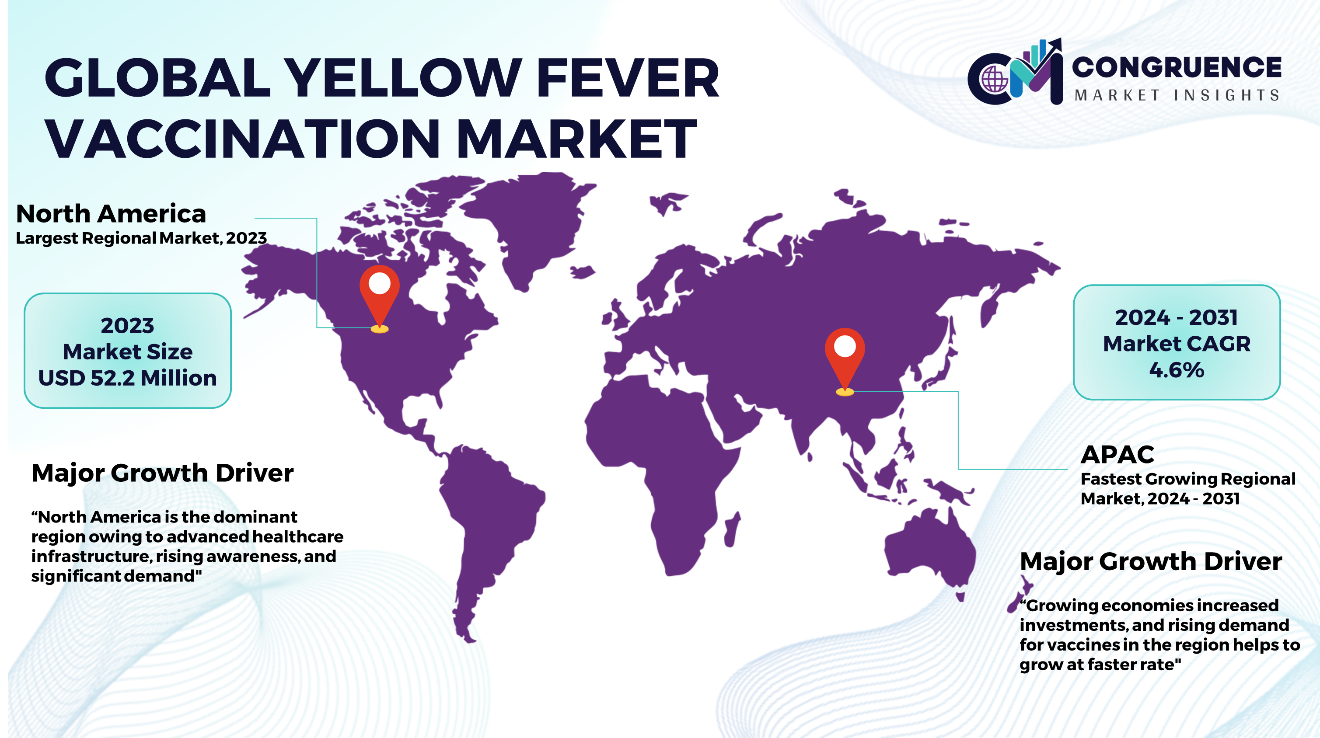
In North America and Europe, the presence of advanced healthcare infrastructure and elevated levels of health consciousness significantly contribute to the robust demand for yellow fever vaccines, especially among travelers venturing into endemic regions. Furthermore, the substantial R&D capabilities within these regions foster continuous innovation in vaccine formulation and delivery systems, ensuring the availability of effective products. Conversely, in the APAC region, the rapid pace of urbanization and concurrent increase in travel activities create environments conducive to disease transmission, thereby escalating the requirement of vaccines. South America contends with intermittent outbreaks, prompting substantial government investments in such programs aimed at controlling the spread of the disease. In the Middle East & Africa, where yellow fever is endemic in specific nations, challenges related to limited healthcare access and intricate infrastructure hinder the efficient distribution and uptake of vaccines.
Market Competition Landscape
The global Yellow Fever Vaccination market is characterized by high degree of competition among a large number of manufacturers. Key players in the Yellow Fever Vaccination market engage in strategies to gain a competitive edge. These strategies include product innovation, pricing strategies, geographic expansion, and the incorporation of sustainable and eco-friendly materials to meet evolving consumer preferences. Established brands leverage their reputation for quality and reliability to maintain market share, while newer entrants focus on disruptive innovations and unique selling propositions.
Key players in the global Yellow Fever Vaccination market implement various organic and inorganic strategies to strengthen and improve their market positioning. Prominent players in the market include:
· Sanofi Pasteur
· GlaxoSmithKline (GSK)
· Merck & Co., Inc. (MSD)
· Johnson & Johnson
· Bharat Biotech
· Emergent BioSolutions
· Takeda Pharmaceutical Company Limited
· Novartis
· Pfizer Inc.
· Sinovac Biotech
· Serum Institute of India
· Bio-Manguinhos/Fiocruz
· Panacea Biotec
· Valneva SE
· CureVac
· Dynavax Technologies Corporation
|
Report Attribute/Metric |
Details |
|
Market Revenue in 2023 |
USD 141.9 Million |
|
Market Revenue in 2031 |
USD 199.0 Million |
|
CAGR (2024 – 2031) |
4.4% |
|
Base Year |
2023 |
|
Forecast Period |
2024 – 2031 |
|
Historical Data |
2019 to 2023 |
|
Forecast Unit |
Value (US$ Mn) |
|
Key Report Deliverable |
Revenue Forecast, Growth Trends, Market Dynamics, Segmental Overview, Regional and Country-wise Analysis, Competition Landscape |
|
Segments Covered |
· By Type (Mild Yellow Fever, and Severe Yellow Fever) · By Vaccine Type (Live attenuated vaccines, Inactivated vaccines, and Others) · By End User (Hospitals, Clinics, and Others) · By Sales Channel (Online Pharmacies, and Offline Pharmacies) |
|
Geographies Covered |
North America: U.S., Canada and Mexico Europe: Germany, France, U.K., Italy, Spain, and Rest of Europe Asia Pacific: China, India, Japan, South Korea, Southeast Asia, and Rest of Asia Pacific South America: Brazil, Argentina, and Rest of Latin America Middle East & Africa: GCC Countries, South Africa, and Rest of Middle East & Africa |
|
Key Players Analyzed |
Sanofi Pasteur, GlaxoSmithKline (GSK), Merck & Co., Inc. (MSD), Johnson & Johnson, Bharat Biotech, Emergent BioSolutions, Takeda Pharmaceutical Company Limited, Novartis, Pfizer Inc., Sinovac Biotech, Serum Institute of India, Bio-Manguinhos/Fiocruz, Panacea Biotec, Valneva SE, CureVac, and Dynavax Technologies Corporation |
|
Customization & Pricing |
Available on Request (10% Customization is Free) |
