Reports
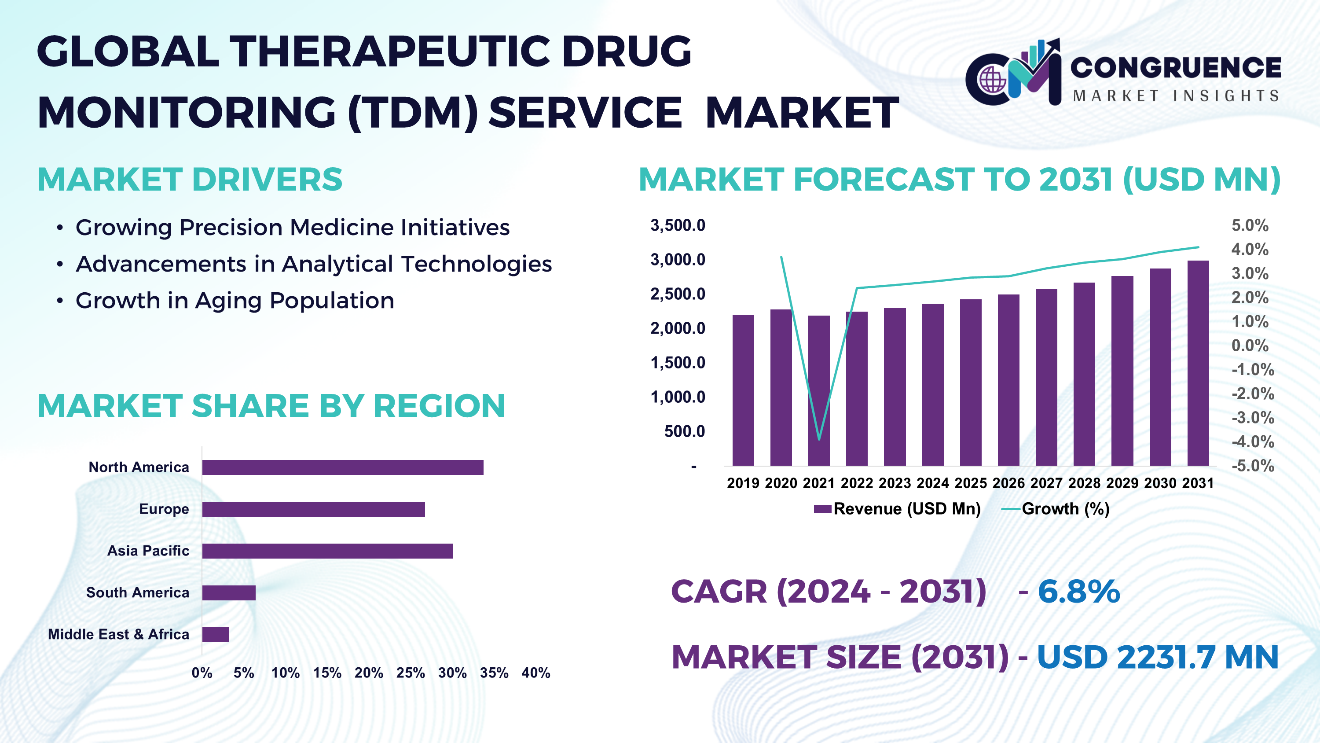
The Global Therapeutic Drug Monitoring (TDM) Service Market was valued at USD 1,316.1 Million in 2023 and is anticipated to reach a value of USD 2,231.7 Million by 2031 expanding at a CAGR of 6.8% between 2024 and 2031.
Therapeutic Drug Monitoring (TDM) is a pivotal practice in optimizing medication efficacy and patient safety by precisely measuring drug concentrations in biological fluids. The technological advancements have revolutionized TDM with the adoption of sophisticated analytical technique such as liquid chromatography-mass spectrometry (LC-MS/MS) and immunoassays, offering improved sensitivity and accuracy in drug level monitoring. These innovations empower healthcare providers to achieve therapeutic targets more effectively, and minimize adverse effects. Furthermore, the increasing utilization of TDM is boosted by the growing awareness of personalized medicine and precision therapeutics, particularly in chronic disease management such as epilepsy, transplant immunosuppression, and infectious diseases. Consequently, there is a notable surge in service requirements, prompting diagnostic laboratories and healthcare facilities to expand TDM capabilities to meet the rising demand. The regulatory authorities, such as FDA, EMA, plays crucial role in establishment of guidelines and standards for TDM practices, ensuring stringent quality assurance and patient safety within the market.
Therapeutic Drug Monitoring (TDM) Service Market Major Driving Forces
Growing Precision Medicine Initiatives: The shift towards personalized medicine and individualized treatment has increased the demand for TDM to optimize the drug dosing and improve patient outcomes. This has led the market to get fueled and generate clientele.
Advancements in Analytical Technologies: As innovations and advancements plays a crucial role in development of the market, here through liquid chromatography-mass spectrometry (LC-MS/MS) and immunoassays, have improved the accuracy rate and efficiency of drug concentration measurements, boosting up the adoption of therapeutic drug monitoring (TDM) Services.
Growth in Aging Population: The aging populations requires the complex medication regimens, making TDM important for monitoring drug levels and preventing adverse drug reactions. Furthermore, helps in reducing the healthcare cost by optimization of drug therapy, and preventing treatment failures, leading to improved healthcare resource utilization.
Therapeutic Drug Monitoring (TDM) Service Market Key Opportunities
Digital Health Integrations: The integration of TDM with digital health platforms and electronic health records (EHRs) will streamline the data management and enable uninterrupted communication. This integration supports the future vision of connected healthcare systems, by enabling comprehensive medication management, and patient-centric care.
Healthcare Industry Expansion: There is a growth in healthcare expenditure and demand for advanced diagnostic service in emerging market owing to rising disposable income and growing health related concerns and awareness. Future growth will involve expanding geographical presence and adapting TDM solutions to diversify healthcare settings, aligning with the vision of accessible and equitable healthcare. This presents untapped opportunities for TDM service providers.
Therapeutic Drug Monitoring (TDM) Service Market Key Trends
· The continued innovation in analytical technologies such as LC-MS/MS and POCT enhances TDM accuracy, efficiency, and accessibility.
· TDM integrates with precision medicine, optimizing drug dosing based on genetic profiles and patient-specific factors.
· Integration with digital health solutions and EHRs streamlines data management, enabling real-time monitoring and personalized medication management.
· Increasing demand for TDM in emerging market drives the geographical expansion and adoption on advanced diagnostic services.
· TDM incorporates pharmacogenomics data for the optimization of drug therapy based on genetic variations, enhancing treatment efficacy.
Region-wise Market Insights
North America accounted for the largest market share at 36.0% in 2023 whereas, Asia Pacific is expected to register the fastest growth, expanding at a CAGR of 7.0% between 2024 and 2031.
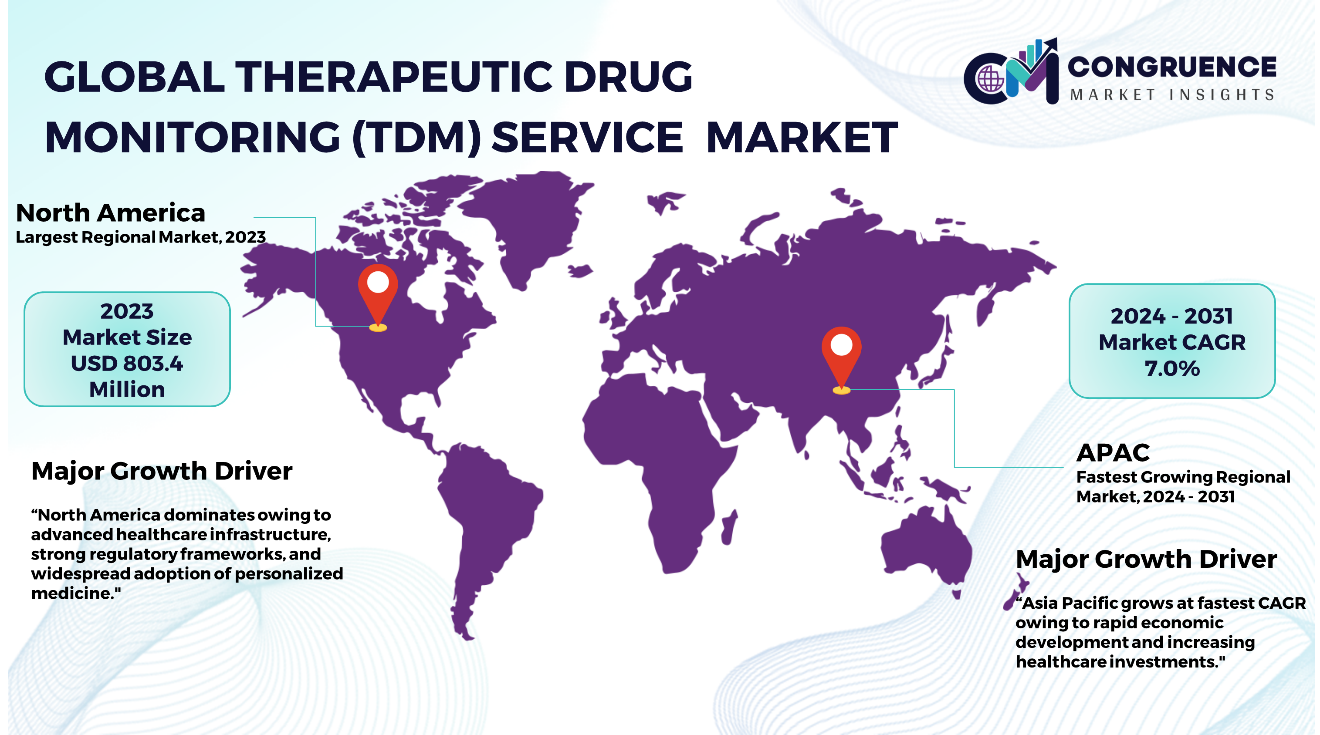
In North America, backed by advanced healthcare infrastructure and a strong focus on personalized medicine, which has led to significant adoption of therapeutic drug monitoring (TDM) services. The region’s robust frameworks play very important role in ensuring adherence to stringent quality standards, thereby fostering market growth. Similarly, Europe demonstrates a commitment to integrating TDM into clinical practice through rigorous regulatory measures. Furthermore, Asia Pacific’s rapid economic development and increased healthcare expenditure create substantial growth opportunities for TDM services. South America poses potential for the growth of the market and seems to be promising for investments for expansion, however, challenges related to regulatory compliance may impact growth dynamics. Middle East & Africa face infrastructure limitations although has steady advance healthcare systems, emphasizing on growth opportunities in near future.
Market Competition Landscape
The global Therapeutic Drug Monitoring (TDM) Service market is characterized by high degree of competition among a large number of manufacturers. Key players in the Therapeutic Drug Monitoring (TDM) Service market engage in strategies aimed at gaining a competitive edge. These strategies include technological innovation, service excellence, geographical coverage, and pricing strategies. Established brands leverage their reputation for quality and reliability to maintain market share, while newer entrants focus on disruptive innovations and unique selling propositions.
Key players in the global Therapeutic Drug Monitoring (TDM) Service market implement various organic and inorganic strategies to strengthen and improve their market positioning. Prominent players in the market include:
· Thermo Fisher Scientific Inc.
· Roche Diagnostics Nederland B.V.
· Siemens Healthcare Private Limited
· Abbott
· Bio-Rad Laboratories, Inc.
· Wieslab Diagnostic Services
· Beckman Coulter, Inc.
· Agilent Technologies, Inc.
· Waters
· Shimadzu
· SEKISUI MEDICAL CO., LTD.
· R-Biopharm AG
|
Report Attribute/Metric |
Details |
|
Market Revenue in 2023 |
USD 1,316.1 Million |
|
Market Revenue in 2031 |
USD 2,231.7 Million |
|
CAGR (2024 – 2031) |
6.8% |
|
Base Year |
2023 |
|
Forecast Period |
2024 – 2031 |
|
Historical Data |
2019 to 2023 |
|
Forecast Unit |
Value (US$ Mn) |
|
Key Report Deliverable |
Revenue Forecast, Growth Trends, Market Dynamics, Segmental Overview, Regional and Country-wise Analysis, Competition Landscape |
|
Segments Covered |
· By Drug Type (Antibiotics, Antiepileptics, Immunosuppressants, Anticoagulants, and Others) · By Application (Infectious Disease, Neurology, Oncology, Cardiology, and Others) · By Testing Service (Routine Therapeutic Drug Monitoring, Specialized Drug Level Testing, and Customized Testing Services) · By End Use Industry (Hospitals, Clinics, Diagnostic Laboratories, and Others) |
|
Geographies Covered |
North America: U.S., Canada and Mexico Europe: Germany, France, U.K., Italy, Spain, and Rest of Europe Asia Pacific: China, India, Japan, South Korea, Southeast Asia, and Rest of Asia Pacific South America: Brazil, Argentina, and Rest of Latin America Middle East & Africa: GCC Countries, South Africa, and Rest of Middle East & Africa |
|
Key Players Analyzed |
Thermo Fisher Scientific Inc., Roche Diagnostics Nederland B.V., Siemens Healthcare Private Limited, Abbott, Bio-Rad Laboratories, Inc., Wieslab Diagnostic Services, Beckman Coulter, Inc., Agilent Technologies, Inc., Waters, Shimadzu, SEKISUI MEDICAL CO., LTD., and R-Biopharm AG |
|
Customization & Pricing |
Available on Request (10% Customization is Free) |
