Reports
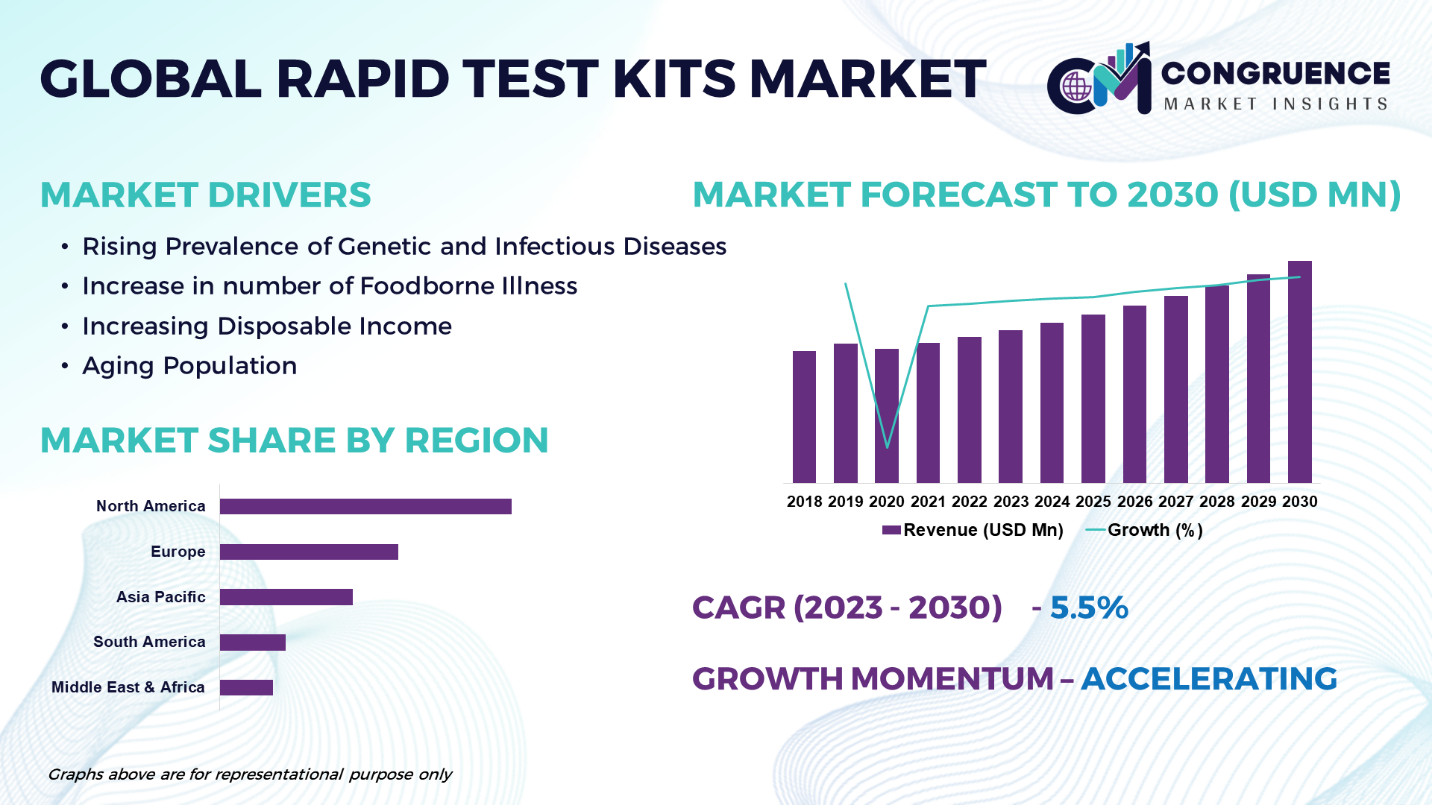
The Global Rapid Test Kits Market is expected to expand at a CAGR of 5.5% between 2023 and 2030. Rapid test kits also known as rapid diagnostic tests are easy to use tests that provide quick result of certain diseases or conditions. Rapid tests are done and provide results at the point of care. A blood sample collected from the patient is applied to the sample pad on the test card along with certain reagents. These test kits aid in the identification and monitoring of underlying illness in living things. Rapid tests are generally used for various purposes, including infectious disease screening, drug testing, pregnancy testing among others. They are classified by their ability to provide results rapidly, usually within minutes, making them suitable tools for immediate medical diagnosis, screening, and surveillance. Although rapid test kits serve the primary purpose of initial screening. The common application of rapid test kits is to diagnose infectious disorders such as HIV, covid-19, malaria, strep throat, and flu. The global rapid test kits market is classified into antigen tests, antibody tests, and other tests. Rapid test kits are commonly used in healthcare settings, including clinics, hospitals, laboratories, and field applications, and in remote areas where access to traditional laboratory facilities may be limited.
Rapid Test Kits Market Major Driving Forces
Rising Prevalence of Genetic and Infectious Diseases: The market is being driven by a rising prevalence of genetic and infectious disease. Rapid tests play a vital role in early detection and treatment and mitigating the spread of diseases.
Increase in number of Foodborne Illness: The rapid increase in the number of foodborne illness is driving demand for rapid test kits. Rapid test kits are commonly used as an efficient screening approach for on-site analysis in a wider range of applications.
Increasing Disposable Income: The rising disposable income coupled with rising living standards especially in developing countries are driving the rapid test kits market growth. With the rising disposable income, the spending power of consumers increases which contributes the market growth.
Aging Population: The increasing aging population in many countries is driving the demand for rapid test kits for diagnosis of age-related conditions, and other diseases. Moreover, the increasing global prevalence of chronic diseases, including diabetes, and cardiovascular conditions has led to a growing demand for rapid test kits for monitoring and management.
Rapid Test Kits Market Key Opportunities
Advancements in Technology: Technological advancements is expected to create lucrative opportunities for the development of more accurate, sensitive, and user-friendly rapid test kits. Advancements in the development of rapid test kits include the introduction of analytical techniques such as nucleic acid amplification tests are expected to offer more efficient solutions.
Rise in Home Healthcare: People are becoming more aware of the importance of health conditions and are using rapid test kits at home in these days are expected to provide growth opportunities for the market during the forecast period. The significant rise in home healthcare and self-monitoring creates an opportunity to develop user-friendly and cost-effective rapid test kits for home use.
Emerging Markets Expansion: Rapid test kits play a vital role in disease screening, and diagnosis. Opportunities lies for manufacturers to expand their presence in the emerging markets where the access to healthcare infrastructure may be limited. Manufacturers can offer affordable and scalable rapid test kits tailored to local needs, contributing to the market growth.
Rapid Test Kits Market Key Trends
· Increasing awareness regarding early diagnosis of disease among people is a key factor shaping the industry growth
· The rising prevalence of infectious diseases is another factor driving the demand for rapid test kits
· The growing need for affordable and accurate diagnostic testing is a notable trend in the market
· Ongoing technological advancements in testing technologies to improve accuracy, sensitivity and portability
· Increased focus on home testing coupled with consumer demand for self-monitoring, and convenience
· The development of user-friendly and accurate home-based testing solutions are gaining momentum
· Multiplex testing that is capable of detecting multiple targets in a single test are gaining popularity due to their cost-effectiveness and efficiency
Market Competition Landscape
The global rapid test kits market is characterized by intense competition among a large number of well-established manufacturers. Major players are focused on the developing advanced, innovative, more accurate, and cost-effective testing solutions to gain a competitive edge. Key players in the rapid test kits market engage in strategies include product innovation, mergers, acquisitions, collaborations, and the incorporation of sustainable and eco-friendly materials to meet evolving consumer preferences. Established brands leverage their reputation for quality and reliability to maintain market share, while newer entrants focus on disruptive innovations and unique selling propositions.
Key players in the global rapid test kits market implement various organic and inorganic strategies to strengthen and improve their market positioning. Prominent players in the market include:
· Abbott Laboratories
· Roche Diagnostics
· Siemens Healthineers
· Becton, Dickinson and Company
· Quidel Corporation
· Danaher Corporation
· Bio-Rad Laboratories, Inc.
· Akers Biosciences, Inc.
· Cepheid, Inc.
· BioMérieux SA
· Meridian Bioscience, Inc.
· OraSure Technologies, Inc.
· Hologic, Inc.
· Cardinal Health
|
Report Attribute/Metric |
Details |
|
Base Year |
2022 |
|
Forecast Period |
2023 – 2030 |
|
Historical Data |
2018 to 2022 |
|
Forecast Unit |
Value (US$ Mn) |
|
Key Report Deliverable |
Revenue Forecast, Growth Trends, Market Dynamics, Segmental Overview, Regional and Country-wise Analysis, Competition Landscape |
|
Segments Covered |
· By Type (Rapid Antigen Testing, Rapid Antibody Testing, and Others) · By Product Type (Over-the-Counter Rapid Testing Kits, and Professional Rapid Testing Kits) · By Application (Infectious Disease Testing, Blood Glucose Testing, Coagulation Testing, Pregnancy and Fertility, Cardiometabolic Testing, and Others) · By End-use (Hospitals & Clinics, Diagnostic Laboratories, Home Care, and Others) |
|
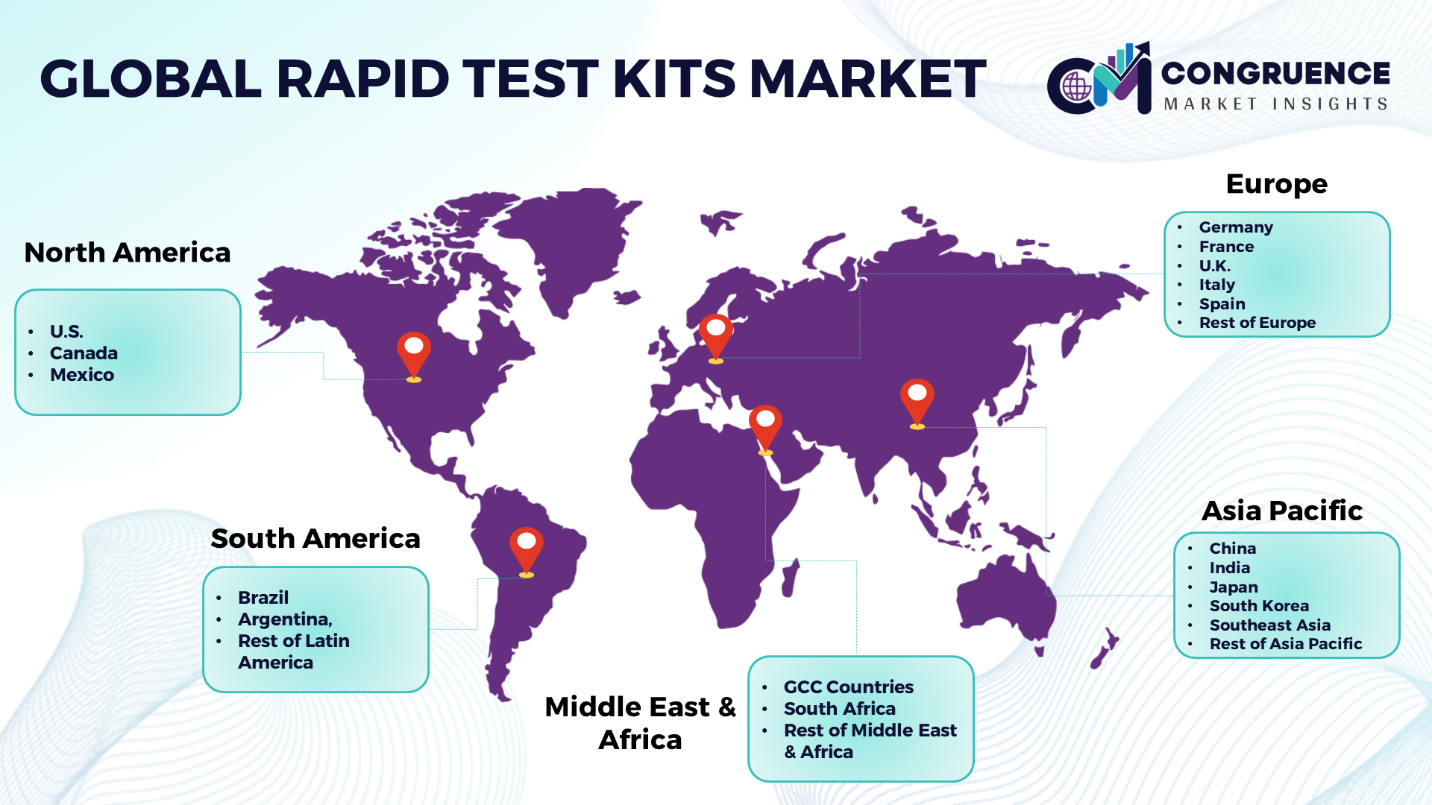
Geographies Covered |
North America: U.S., Canada and Mexico Europe: Germany, France, U.K., Italy, Spain, and Rest of Europe Asia Pacific: China, India, Japan, South Korea, Southeast Asia, and Rest of Asia Pacific South America: Brazil, Argentina, and Rest of Latin America Middle East & Africa: GCC Countries, South Africa, and Rest of Middle East & Africa |
|
Key Players Analyzed |
Abbott Laboratories, Roche Diagnostics,Siemens Healthineers,Becton, Dickinson and Company,Quidel Corporation,Danaher Corporation,Bio-Rad Laboratories, Inc.,Akers Biosciences, Inc.,Cepheid, Inc.,BioMérieux SA,Meridian Bioscience, Inc.,OraSure Technologies, Inc.,Hologic, Inc., and Cardinal Health |
|
Customization & Pricing |
Available on Request (10% Customization is Free) |
